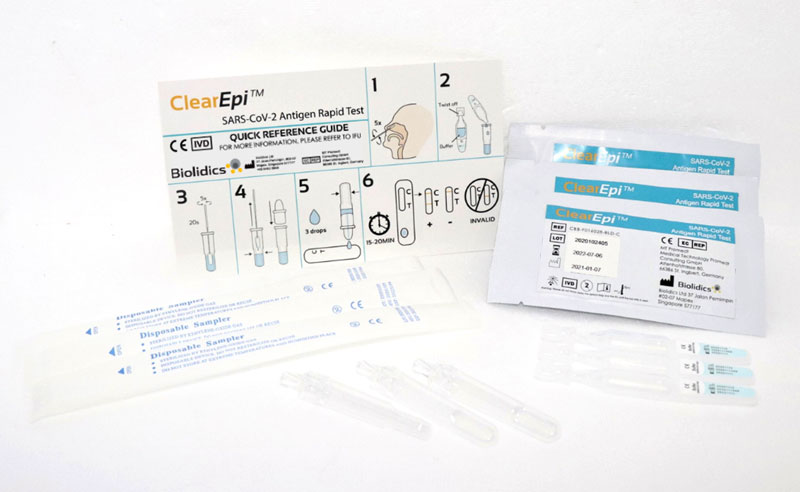
ClearEpi™ SARS-CoV-2 Antigen Rapid Test
Catalogue No.: CBB-F016028-BLD-C

- Qualitative detection of SARS-CoV-2 nucleocapsid antigen in nasal specimen directly from individuals suspected of COVID-19 infection
- Point of care testing with no additional equipment required
- Convenient storage conditions : 2 - 30°C
- High sensitivity, specificity and accuracy
ACCURACY OVER 98%
CLINICAL DATA PERFORMANCE
The kit showed 98.15% of sensitivity and 98.75% of specificity.
| Reagent Test Results | RT-PCR Comparator | Subtotal | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 53 | 2 | 55 |
| Negative | 1 | 158 | 159 |
| Subtotal | 54 | 160 | 214 |
Positive Percent Agreement (PPA) = 53/54 (98.15%) (95%CI: 90.1%~100.0%)
Negative Percent Agreement (NPA) = 158/160 (98.75%) (95%CI: 95.6%~99.8%)
Accuracy = (53+158)/214×100% = 98.60%
Antigen Test - LFA
Catalogue No.: CBB-F016026
Clinical Performance
An in vitro immunochromatographic assay for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in nasal swab specimens.
Clinical Study Results From Symptom Onset
| Reagent Test Results | PCR Comparator | Subtotal | |
| Positive | Negative | ||
| Positive | 77 | 3 | 80 |
| Negative | 1 | 109 | 110 |
| Subtotal | 78 | 112 | 190 |
- Positive Percent Agreement (PPA) = 77/78(98.72%) (95%CI:93.0%~100%)
- Negative Percent Agreement (NPA) = 109/112(97.32%) (95%CI:92.4%~99.4%)
- Accuracy = (77+109)/190x100% = 97.89%
- Kappa = 2x8390/17450 = 0.96>0.5